Fine Ceramics, sometimes referred to as "advanced ceramics," are engineered materials that support the development of cutting-edge technology.
Electricity and Magnetism (3)
Dielectricity to Accumulate Electricity
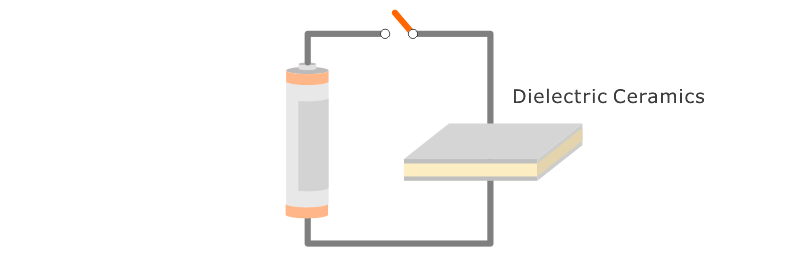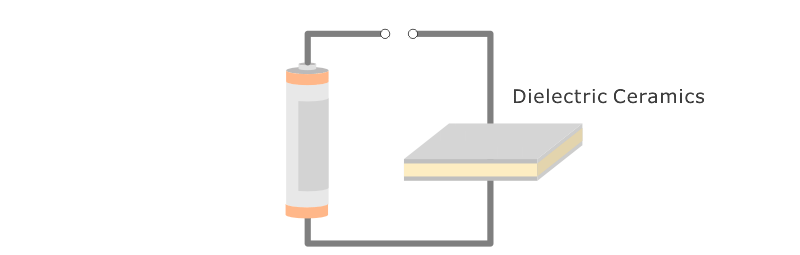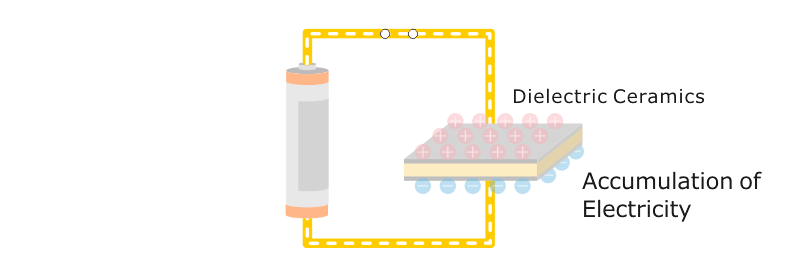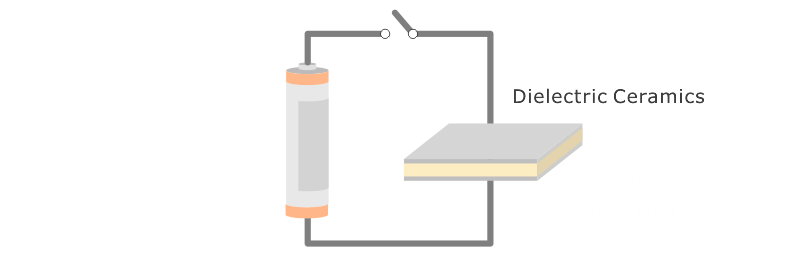
When voltage is applied to electrodes attached to opposite sides of an insulating ceramic, they exhibit electricity-accumulating properties. These insulators are said to be dielectric.
An insulator that can accumulate a volume of electricity exceptionally well is called ferroelectric. Some Fine Ceramics (also known as "advanced ceramics") display this property. Consequently, ceramic has become an indispensable material for producing capacitors and electronic components that are widely used in products such as computers, televisions and mobile phones. Capacitors serve as "traffic controllers" within an electronic circuit by conducting electricity to certain parts, temporarily blocking electricity, or blocking only certain types of electrical signals.
Application: Ceramic capacitors.
Description
Dielectricity
Electricity, by definition, is the movement of electrons within the molecules of a material. Although electrons can move freely within conductors, they cannot do so within insulators. When a direct current voltage is applied to an insulator, electrons do not separate from molecules, and are divided by an electrical charge (positive or negative) induced at both ends of the material.
In addition, ions themselves may move. Materials in which electrical charges appear at both ends are called dielectric substances. Dielectricity is measured by the relative dielectric constant, a value representing the ratio of the dielectric constant of the material in question and that of a vacuum. The dielectric constant of quartz is 3.8, while that of sapphire (main component: oxidized aluminum) is 9.4. The dielectric constant of barium titanate, a ferroelectric material, is as high as 4,000 to 5,000.
Relative Dielectric Constants
| Quartz (SiO2) | 3.8 |
|---|---|
| Sapphire (Al2O3) | 9.4 |
| Barium Titanate (BaTiO3) | 4,000 – 5,000 |
The term "Fine Ceramics" is interchangeable with "advanced ceramics," "technical ceramics" and "engineered ceramics." Use varies by region and industry.
People who read this page also read.
Electricity and Magnetism (1)
Electricity and Magnetism (1)
Electrical Insulation to Inhibit Electricity from Passing Through
Electrical Insulation to Inhibit Electricity from Passing Through
Characteristics of Fine Ceramics

Electricity and Magnetism (2)
Electricity and Magnetism (2)
Conductivity to Allow Electricity to Pass Through
Conductivity to Allow Electricity to Pass Through
Characteristics of Fine Ceramics

Electricity and Magnetism (4)
Electricity and Magnetism (4)
Piezoelectricity to Convert Electricity into Power/Power into Electricity
Piezoelectricity to Convert Electricity into Power/Power into Electricity
Characteristics of Fine Ceramics

Electricity and Magnetism (5)
Electricity and Magnetism (5)
Magnetism to Hold Magnetic Force
Magnetism to Hold Magnetic Force
Characteristics of Fine Ceramics

Different Types of Fine Ceramics
Different Types of Fine Ceramics
Wide Variety of Products to Support both Industry and Society
Wide Variety of Products to Support both Industry and Society
Introduction to Fine Ceramics
If you want to use ceramics in business, click here.
Kyocera's Fine Ceramics products (All websites below open in a separate window.)
Product Category
 Semiconductor / LCD Processing Equipment
Semiconductor / LCD Processing Equipment
 Life / Culture / Industrial Machines
Life / Culture / Industrial Machines
 Wireless Communications
Wireless Communications
 Computer Peripherals
Computer Peripherals
 Environmental Preservation / Renewable Energy
Environmental Preservation / Renewable Energy
 Medical Equipment / Devices
Medical Equipment / Devices
 Single-Crystal Sapphire Products
Single-Crystal Sapphire Products
 Metallized / Vacuum Components
Metallized / Vacuum Components
 Electronics Industry
Electronics Industry
 Heaters
Heaters
 Piezoelectric Ceramics
Piezoelectric Ceramics
Search by Material
 Alumina
Alumina
 Silicon Nitride
Silicon Nitride
 Silicon Carbide
Silicon Carbide
 Sapphire
Sapphire
 Zirconia
Zirconia
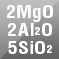 Cordierite
Cordierite
 Yttria
Yttria
 Aluminum Nitride
Aluminum Nitride
 Cermet
Cermet
 Mullite
Mullite
 Steatite
Steatite
 Forsterite
Forsterite
Search by Property/Characteristic


- Thermal Properties
- Coefficient of Thermal Expansion
- Thermal Conductivity
- Heat Shock Resistance

- Electrical Properties
- Insulation / Semiconductivity

- Chemical Properties
- Chemical Resistance