
AG-PROTEX


AG-PROTEX is approved or cleared for sale in the Japanese market only.
"AG-PROTEX" is a trademark of KYOCERA Corporation.
Surgical site infections (SSIs) of bones and joints are one of the three major complications of prosthetic joint replacement, often making the treatment difficult, which can recur and lead to limb amputation.
The incidence rates of deep SSI are as follows: (Figure 1) (Literature 1)

Figure 1. The incidence of deep SSI
According to the results of a survey conducted between January and December 2004, at 2,241 training facilities certified by the Japanese Orthopaedic Association the incidence of SSI was reported to be 134 out of 9,882 (1.36%) patients undergoing primary joint replacement.
MRSA and MRSE, which are drug-resistant bacteria, accounted for 46% of the pathogens. (Table 1) (Literature 2)
(MRSA : methicillin-resistant Staphylococcus aureus, MRSE : methicillin-resistant Staphylococcus epidermidis)
| SSI of artificial joint replacement(134 cases) |
|
|---|---|
| MRSA(Methicillin-resistant Staphylococcus aureus) | 56 cases (42%) |
| Staphylococcus aureus | 23 (17%) |
| Staphylococcus epidermidis | 15 (11%) |
| Pseudomonas aeruginosa | 5 (4%) |
| MRSE (Methicillin-resistant Staphylococcus epidermidis) | 5 (4%) |
| Escherichia coli | 5 (4%) |
| Anaerobic bacteria | 3 (2%) |
| Others | 10 (8%) |
| Unknown | 12 (9%) |
Table 1. Organisms causing SSI in prosthetic joint replacement
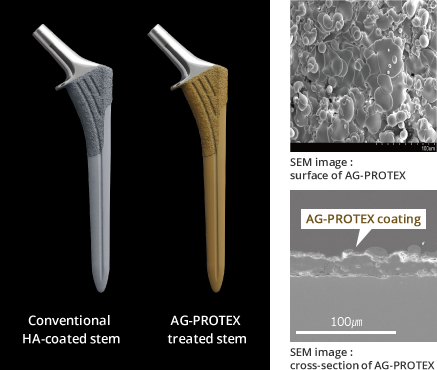
Figure 2. Surface/cross-sectional SEM image of AG-PROTEX
Kyocera's HA coating has been applied to the bone bonding surface of cementless artificial joints and has been in clinical use for more than 10 years. (Literature 3) This time, we have developed our proprietary thermal spray technology to maintain osteoconductivity of the HA coating and add antibacterial properties of silver. (Literature 4)
AG-PROTEX succeeded for the first time in achieving both the antibacterial properties of silver and osteoconductivity / fixation of HA.
AG-PROTEX is applied to the bone bonding section of the cementless prosthesis to provide antibacterial, osteoconductive, and bone fixation properties to the surfaces of implants.
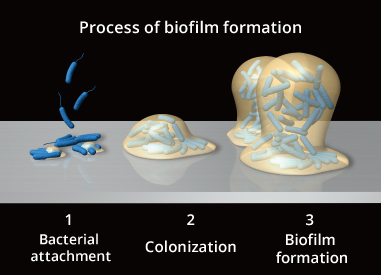
Figure 3. Process Image of Biofilm Formation
SSIs related to prosthetic surgery are called implant-related infections, or Peri-prosthetic joint infection (PJI),
and are known to involve biofilms in the onset and/or exacerbation of infection. (Literature 5)
Bacteria in biofilms are difficult to treat by antibiotics and the increase of drug-resistant bacteria, such as MRSA, make infection treatment difficult.
AG-PROTEX elutes silver ions, and reduces bacterial adhesion to AG-PROTEX surfaces due their bactericidal properties. As a result, AG-PROTEX demonstrates an inhibitory effect on surface colonization and biofilm formation*.
i.e., it is expected to inhibit the early stages of biofilm formation.
*Confirmed by in vitro test
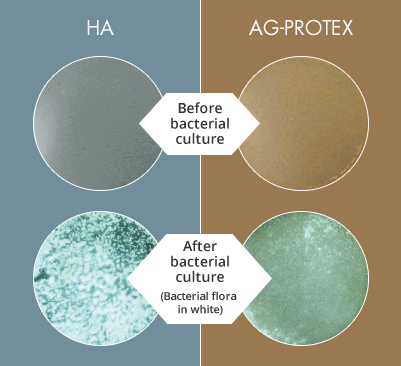
Figure 4. Results of Bacterial Adhesion Inhibition Test
When AG-PROTEX’s bacterial adherence inhibition qualities were evaluated, HA surfaces were covered with bacteria (MRSA, white part) and the bacterial coverage ratio was 88%. On the other hand, the bacterial coverage on the surface of AG-PROTEX was as low as 9% confirming that AG-PROTEX has bacterial adhesion inhibition effect(Figure 4). (Literature 6)
AG-PROTEX and HA specimens (20mm in diameter) were inoculated with 105CFU (colony-forming units) of MRSA, incubated at 37℃ for 24 hours, and washed with PBS (phosphate-buffered saline) to flush the floating bacteria. After the washed specimens were dried, the colonies of viable bacteria on the specimens were observed under a light microscope, and image analysis was performed to calculate the bacterial coverage ratio (percentage of the surface specimen covered with a colony of viable bacteria). The white part in Figure 4 is the bacterial colony.
When the ability of AG-PROTEX to inhibit biofilm formation was evaluated, the mean biofilm coverage rate (BCR) was 21.2% on HA surfaces after one day of incubation. On the other hand, the surface of AG-PROTEX was 8.8%.
Although AG-PROTEX did not show significant reduction of BCR compared to HA in a 3-day culture period, AG-PROTEX was found to show significant reduction of BCR compared to HA excluding the 3-day culture period. Therefore, based on the trends in the results of the entire study in each culture period, AG-PROTEX was considered to have an inhibitory effect on biofilm formation for up to 28 days, including 3 days in each culture period (Figure. 5).
For the 3-day incubation results, the biofilm coverage on HA surface tended to be relatively low and different compared to the trends in the other culture period results, but the cause was difficult to identify.
Additional studies with only 3 days of incubation confirmed that the BCR of AG-PROTEX was statistically significantly lower than that of HA.
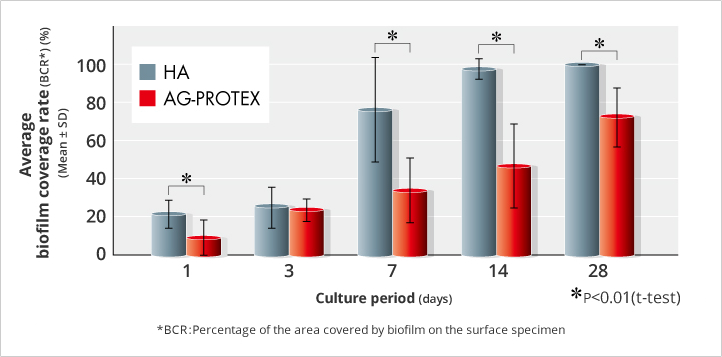
Figure 5. Results of Biofilm Formation Inhibition Test (in-house data) (n=35)
AG-PROTEX and HA specimens (14mm in diameter) were inoculated with 105CFU of MRSA, incubated at 37℃ for 1 hour, and washed with PBS to flush the floating bacteria. 2 or 3 copies of each specimen were then placed in a petri dish (90mm in diameter) containing 15mL bovine serum and incubated with stirring (60rpm) at 37℃ for up to 28 days. The medium was changed every 2-3 days during the incubation period. After a given incubation period, the test specimens were collected, washed with PBS to wash off the floating bacteria, and the biofilm was stained with fluorescent stain. Biofilms on the specimen surface were observed by fluorescence microscopy, and image analysis was performed to calculate the BCR.
In vitro antibacterial tests based on the Japanese Industrial Standard (JIS) confirmed its antibacterial properties against the following 6 bacteria that cause infection. In addition, as a result of the 14-day study, it was confirmed that AG-PROTEX has persistent antibacterial activity. (Table 2) (Literatures 2 and 7)
| Bacterial name | Antibacterial properties |
|---|---|
| + | |
| + | |
| + | |
| + | |
| + | |
| + |
Table 2. Confirmed Bacteria for AG-PROTEX Anti-Bacterial Properties
Antibacterial test of film-adhesion method was carried out referring to the JIS Z 2801 standard. The AG-PROTEX and HA specimens (50mm longitudinally, 50mm laterally) were inoculated with 105 CFU of bacteria, and the surfaces of the specimens were covered with polyethylene films (40mm longitudinally, 40mm laterally) and cultured at 37℃ for 24 hours. After incubation, viable bacteria on the test piece and the film were collected using vortex. After the number of viable bacteria was determined by the plate method, the antibacterial activity value (R) was calculated according with the following formula.
R = (average value of logarithm of the number of viable bacteria on HA specimens)-(average value of logarithm of the number of viable bacteria on AG-PROTEX specimens)
Based on the JIS Z 2801 standard, when R was 2 or more, it was determined to be as "antibacterial". An antibacterial activity value of 2 or more means that bacteria bacterium are killed by 99% or more.
The antibacterial properties of AG-PROTEX in bones were evaluated, the number of viable bacteria of AG-PROTEX was significantly lower than that of HA between 24 to 72 hours after surgery, demonstrating that the antibacterial activity of AG-PROTEX even in vivo (Figure. 6).
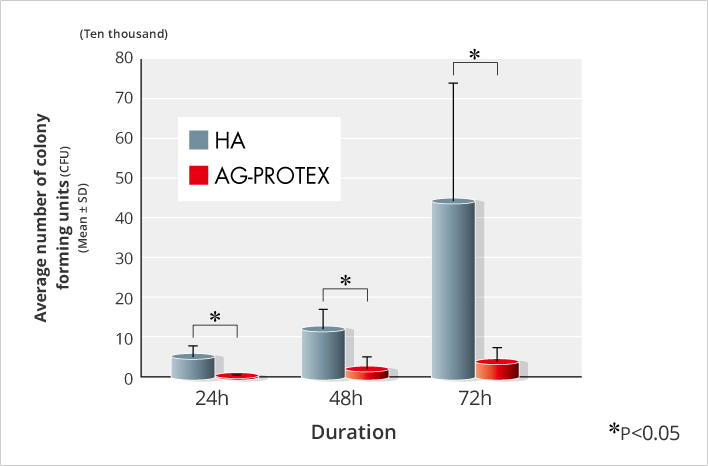
Figure 6. Results of Intraosseous Antibacterial Test (n=5)
After drilling in the tibial medullary of ten-week-old Sprague-Dawley rats , 102 CFU of MRSA fluid was injected into the drilled hole, and the hole was closed after inserting AG-PROTEX and HA specimens (1mm in diameter, 20mm in length). At 24, 48, and 72 hours after surgery, the tibial bone containing the test specimens was removed, and viable bacteria were collected by pulverization and ultrasonication, and the number of the viable bacteria was determined by the plate method.
Based on the above results, it is confirmed that AG-PROTEX inhibits bacterial adhesion and bacterial biofilm formation by including silver, which has a broad antibacterial spectrum in the coating, and is expected to show the antibacterial effect in vivo.
As a result of the push-out test, it was confirmed that AG-PROTEX has the same intraosseous fixation force as HA (Figure. 7).
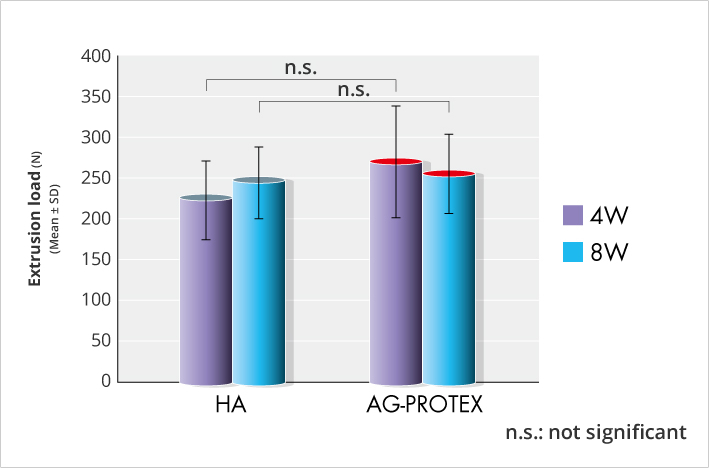
Figure 7. Results of intraosseous fixation force test (in-house data) (n=7 or 8)
After drilling a hole in the diaphysis of the femur of 7- to 9-week-old Japanese white rabbits, the AG-PROTEX and HA specimens were implanted in the holes perpendicular to the long axis of the femur. The femoral region including the specimen was collected in postoperative 4 weeks and 8 weeks, and the push-out load was evaluated in the push-out test. That is to say, the femoral part was fixed in a special jig, and the load was applied to the test piece using the universal test machine at the constant speed, and the maximum push-out load in which the fixation in the bone of the test piece fractured was measured.
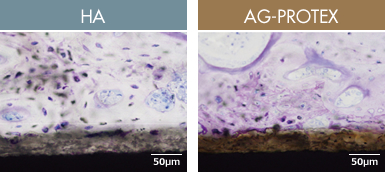
Figure 8. Tissue observation image at 4 weeks after implantation (in-house data)
When the osteoconductivity of AG-PROTEX was evaluated in a bone implant test in rabbits, new bone was formed on the surface of the coated layer for both HA and AG-PROTEX, and the bone connected sites were also often observed. Therefore, AG-PROTEX is considered to have excellent osteoconductivity as well as HA (Figure. 8).
After drilling holes in the tibial shaft of 7-to 9-week-old Japanese white rabbits, AG-PROTEX and HA specimens (columnar specimens coated on the columnar surface) were implanted.
The tibia, including the specimen, was collected in postoperative 4 weeks, and the decalcified sample with the resin embedding was prepared.
After the toluidine blue dyeing, it was observed by light microscope.
These results confirm that AG-PROTEX has bone osteoconductivity and bone fixation properties comparable to those of conventional HA coating, and it is expected that AG-PROTEX has a combination of silver antibacterial properties, osteoconducivity, and bone fixation properties in vivo.
The Ministry of Health, Labor and Welfare (MHLW) issued the "Basic Principles of Biological Safety Evaluation required for Applications of Manufacturing and Marketing Approval of Medical Devices" (PFSB/ELD Notification No. 0301-20 dated March 1, 2012).
Biological safety tests of AG-PROTEX conducted in accordance with this Notification and the International Standards ISO10993-1 2009. No toxicity was observed in any of the following tests (Table 3).
| Test Items performed | Test results |
|---|---|
| Cytotoxicity test | No toxicity |
| Irritation test (intradermal reaction) | Conformance |
| Skin sensitization test | No skin sensitization |
| Genotoxicity test (reverse mutation) | Negative |
| Genotoxicity test (chromosomal aberrations) | Negative |
| Systemic toxicity test (acute and subacute) | No toxicity |
| Implantation test (intraosseous) | No tissue damage shown |
Table 3. Results of Biological Safety Test(in-house data)
Silver concentrations in the sera of 20 patients who underwent primary total hip arthroplasty using products treated with AG-PROTEX were measured. The median silver concentrations peaked at 2 weeks after surgery and gradually decreased (Figure. 9).
There were no problems attributable to argyria or products during the follow-up period.
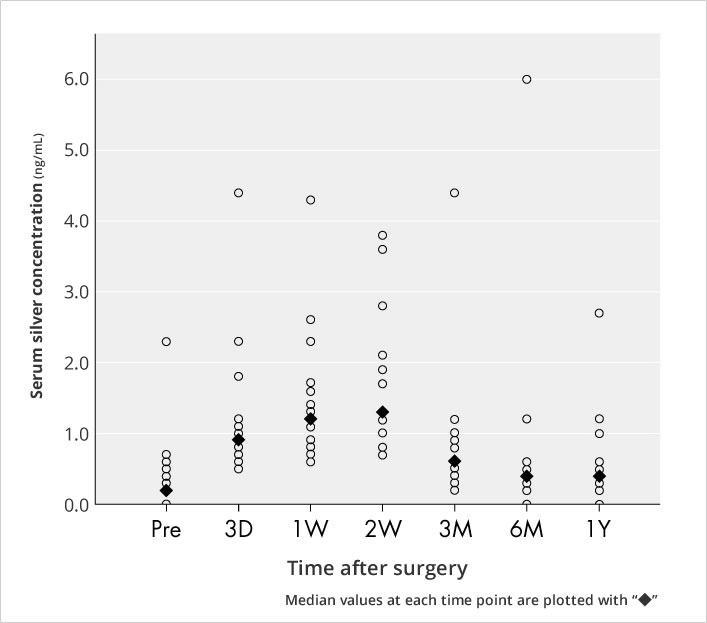
Figure 9. Serum Silver Concentration
Based on the results of antibacterial tests, osteoconductivity tests, and safety tests of AG-PROTEX, we believe that AG-PROTEX is a safe surface-treatment technology similar to conventional products while also realizing both antibacterial properties and osteoconductivity.